Rózsa Balázs előadása (2017. 10. 26.)
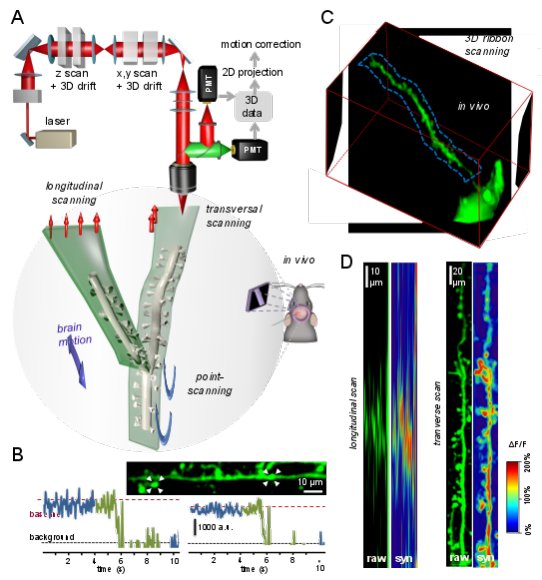
Fast 3D imaging of neuronal and dendritic spine assemblies in the visual cortex to understand neuronal computation in behaving animalsOur long-term aim is to assess the feasibility of creating an “artificial sense” and, thereby, a possible sensory (visual) prosthetic. While working towards this goal, we will have to address the question of how neural assembly activity relates to subjective perceptions. Finding and understanding these functional assemblies will make it possible to reactivate them in a precise, biologically relevant manner to elicit similar cortical activation as visual stimulation. Recent publications suggest that cortical connectivity can be mapped by two-photon microscopy. Three-dimensional (3D) random-access point scanning can simultaneously read out neural activity on both the somatic and dendritic scales which is required to map coding assemblies in the visual cortex. This method can increase measurement speed and signal-to-noise ratio (SNR) by several orders of magnitude, but suffers from one main disadvantage: fluorescence information is lost during brain movement. Therefore we developed a novel 3D microscope technique for high-throughput assembly mapping in behaving animals. The novel 3D DRIFT acousto-optical scanning microscopy can extend each scanning point to small 3D lines or surface or volume elements, preserving fluorescence information for motion correction. The method effectively eliminates in vivo motion artifacts, allowing fast 3D measurement of over 150 dendritic spines with 3D lines, over 100 somata with squares and cubes, or multiple spiny dendritic segments with surface and volume elements in behaving animals. Finally, a four-fold improvement in total excitation efficiency resulted in about 500 µm × 500 µm × 650 µm, scanning volume with GECIs. Using the new microscope we mapped activity of large neuronal assemblies associated with different visual stimulation.
Vissza az előadásokhoz